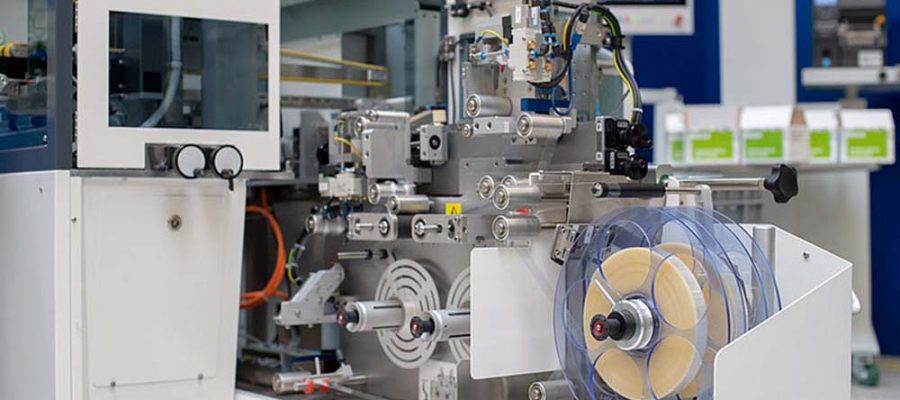
Print, seal and check! Tamper proof labeling 2.0 with Weber and Traxeed. Traxeed is an expert in aggregation and data transfer at all levels of automation. More than 100 employees develop, manufacture, install and validate one-stop systems for tracking medicines. Since the approval of the EU fraud proof packaging directive, Traxeed and Weber Marking Systems have been working on the perfect tamper proof labeling solution.
Tamper proof challenges
When Weber Marking Systems and Traxeed started cooperation, their goal was to conceive a labeling solution that could offer the following:
- Space-saving tamper-evident labeling module
- Labeling 5 products per second
- Easy maintenance and refilling of label rolls
- Reliable protection against tampering
Together, Weber Marking Systems and Traxeed have developed the Traxeed ItemUnit-Tamper Evident: a complete tamper proof solution for easy integration into pharmaceutical companies’ existing production facilities. One machine that develops, prints, seals and checks.

Features of our new tamper proof labeling machine
- Compact installation width of 300 mm
- Plain text & 2D data matrix code printing
- Manipulation safety through security seals
- Label inspection with cameras and sensors
- The labeling machine can be extended for maintenance
How our tamper proof labeling machine works in practice
Fase 1 – Printing

There are two steps to making pharmaceutical packaging tamper proof: First, the EU fraud proof packaging directive requires folding cartons to be labeled with a GTIN (Global Trade Item Number), a serial number, a batch number and an expiry date. The information is printed as plain text and also encoded as a two-dimensional data matrix code. The Traxeed ItemUnit-Tamper Evident receives the print data for each job from a superior site management system called Multi Line Manager. It labels more than 5 products per second at a belt speed of up to 60 metres per minute. After the information is printed as plain text and code, a camera creates the security that actually prints with the data sent to the printer.
Fase 2 – Sealing

The anti-counterfeiting device is the second part of preventing counterfeiting. At a glance, the untouched perforation of the security seals can be seen, which makes it possible to determine whether the packaging has already been opened. This is why the ItemUnit-Tamper Evident then seals folding cartons with Tamper Evident seals. The cartons pass through the Tamper Evident labeling machine, which – without making contact – applies transparent plastic seals to both sides of the carton. One half of the micro-perforated label sticks out and is folded exactly over the edge of the carton by a guide plate. That may sound simple, but with more than 300 folding boxes per minute faultlessly, it’s quite a piece of work!
Fase 3 – Inspection

Incorrectly labeled medicine packages are not allowed to leave production. Cameras and sensors are used to check labeling due to the production speed common in the pharmaceutical industry. Sensors in the Traxeed ItemUnit-Tamper Evident check the gloss of the surface to determine whether the package is correctly labeled. If this is not the case, the unit is taken off the line by a blast of air.
Once the packages have been labeled, the ItemUnit-Tamper Evident tells the Multi Line Manager exactly how many serial numbers were printed. The data is transmitted to the EU hub, either via a business and planning logistics system (e.g. Arvato CSDB or other systems such as SAP) or manually via the EMVO gateway. The hub sends the serial numbers to the verification systems (NVS) of the countries where the products are sold. When a product is sold, for example in a pharmacy, the employee scans the data matrix and is immediately told by the NVS that the product is authentic. The serial numbers on the boxes confirm this with the NVS databases.
Our tamper-proof solutions help prevent counterfeiting
The safety of medicines is of vital importance to consumers and manufacturers alike. Therefore, product traceability and proof of first opening are essential throughout the production and logistics process in the pharmaceutical industry to prevent counterfeiting.
Weber Marking Systems manufactures coding and labeling solutions for the primary, secondary and tertiary packaging of medicines which comply with EU Directive 2011/62 for tamper-evident packaging.
PHARMA SOLUTIONS